​ according to brown, et al., what is the culture of honor hypothesis?

Human ARF Specifically Inhibits Epimorphic Regeneration in the Zebrafish Center
one
Department of Surgery, Division of Plastic Surgery, Program in Craniofacial Biology, University of California, San Francisco, CA 94143, U.s.a.
two
Department of Surgery and Orofacial Sciences, Program in Craniofacial Biology, Eli and Edythe Wide Center of Regeneration Medicine and Stem Cell Research, University of California, San Francisco, CA 94143, USA
three
Section of Surgery, Division of Plastic Surgery, University of Wisconsin Schoolhouse of Medicine and Public Health, Madison, WI 53726, Us
4
Department of Surgery and Orofacial Sciences, Division of Plastic Surgery, Plan in Craniofacial Biology, University of California, San Francisco, CA 94143, U.s.
5
Edythe Wide Center of Regeneration Medicine and Stalk Cell Research, University of California, San Francisco, CA 94143, USA
*
Author to whom correspondence should exist addressed.
Received: 29 May 2020 / Revised: 13 June 2020 / Accustomed: sixteen June 2020 / Published: eighteen June 2020
Abstruse
The Alternative Reading Frame (ARF) poly peptide is a tumor suppressor encoded by the Cyclin Dependent Kinase Inhibitor 2A gene in mammals but not lower regenerative vertebrates, and has been previously implicated equally a context-sensitive suppressor of regeneration in murine skeletal musculus and humanized ARF-expressing zebrafish fins. This study extends our investigation of the role of ARF in the regeneration of other solid tissues, including the zebrafish heart and the mammalian digit. Centre regeneration after cryoinjury was used to mimic massive myocardial infarction. ARF gene expression was upregulated during the cardiac regenerative process and slowed the rate of morphological recovery. ARF specifically impacts cardiomyocytes, neovascularization, and the endothelial-mesenchymal transition, while not affecting epicardial proliferation. This suggests that in the context of regeneration, ARF is specifically expressed in cells undergoing dedifferentiation. To investigate ARF as a suppressor of epimorphic regeneration in mammalian systems, we also tested whether the absence of ARF was permissive for murine digit regeneration, merely constitute that ARF absence lone was insufficient to significantly alter digit restoration. These findings provide additional evidence that ARF suppresses epimorphic regeneration, but suggests that modulation of ARF lonely is insufficient to permit regeneration.
1. Introduction
Humans do non undergo epimorphic regeneration after significant injury, which instead leads to collagen degradation, scar formation, and functional impairment. In contrast, zebrafish are able to fully regenerate circuitous structures such every bit the fin and eye even after massive injury from fin amputation [one], ventricular resection [two,3], genetic ablation [iv], hypoxia-reoxygenation injury [5], and ventricular cryoinjury [6,vii,8]. Zebrafish have thus served as excellent models to advance our knowledge of epimorphic regeneration and also elucidate limitations to human regeneration.
Tumor suppressor genes have increasing importance in cancer protection manifested nearly strongly in mammalian phyla, but may also adversely affect mammalian regeneration [ix]. The human Culling Reading Frame (ARF) gene product is an unusual tumor suppressor differentiated from others because it does not take whatsoever orthologs represented in highly-regenerative species [10,11], suggesting that the absence of ARF may be permissive for regeneration in those species. ARF is a protein encoded by Cyclin dependent kinase inhibitor 2A (Cdkn2a) [12] that responds to inappropriate retinoblastoma (Rb) pathway signaling [13] by sequestering Mouse double minute 2 (MDM2) to regulate Tumor poly peptide 53 (p53) [xiv,15] and promoting jail cell cycle arrest or apoptosis to maintain the postmitotic state. Prior studies accept implicated ARF as a regeneration suppressor in vitro by showing that blocking ARF in the context of compromise of the Rb pathway results in dedifferentiation and proliferation of mammalian muscle cells in culture [16]. In vivo in humanized ARF transgenic zebrafish, it was demonstrated that ARF is selectively activated during regenerative contexts to inhibit epimorphic fin regeneration later on fin amputation [17]. Thus, experimental evidence supports the concept that ARF is a regeneration suppressor.
Although epimorphic regeneration exists in diverse tissues and a limited number of species, it is unknown how conserved epimorphic regeneration is mechanistically in different tissue and jail cell types. Likewise, information technology is unclear how generalizable are the mechanistic underpinnings of putative regeneration suppression by ARF, and by extension how dependent epimorphic regeneration is on the dedifferentiation of postmitotic cells. Zebrafish fin regeneration differs from cardiac regeneration at the histological and tissue levels. An amputated fin has a sharply demarcated wound border that regenerates primarily using a blastema, a heterogenous pool of highly proliferative mesenchymal cells [two,18,nineteen]. By comparison, the hallmarks of cardiac regeneration are remuscularization, angiogenesis, immunological remainder, resolution of fibrosis, and electromechanical stability stemming from multiple tissue types rather than a atypical blastema [20]. Cardiac cryoinjury in zebrafish results in immediate massive localized damage, and fibrotic tissue deposition like to scarring after myocardial infarction in mammals [half-dozen,seven]. All major cardiac tissue types including the epicardium, endocardium, and myocardium are then activated in an organ-wide response to the injury [21,22]. Epicardial cells throughout the heart initially induce developmental markers T-Box transcription cistron 18 (tbx18), retinaldehyde dehydrogenase 2 (raldh2), fibronectin 1 (fn1), and Wilm's tumor 1 (wt1), and undergo rapid proliferation to environment the exposed myocardium [21,23]. Activated epicardium nearly the injury site upregulates Sonic hedgehog (Shh), Notch, platelet-derived growth cistron receptor β (pdgfrβ), insulin-like growth factor (igf2b), Bone morphogenetic protein (Bmp), Transforming growth factor β (TGFβ), and fibroblast growth factor (fgfr) to stimulate neighboring cardiomyocyte proliferation [iii,21,23,24]. Epicardial cells also promote vasculature regeneration indirectly through paracrine mechanisms [25], although a subset undergoes epithelial-mesenchymal transition (EMT) and generates new vasculature directly [21]. Endocardial cells likewise upregulate raldh2 as an initial response to injury [26], and after contribute to cardiomyocyte regeneration [24]. Activated endocardial cells have besides demonstrated an power to induce atrial to ventricular myocardial transdifferentiation through the Notch pathway [27]. Cardiac myosin calorie-free chain 2 (Cmlc2+) cardiomyocyte proliferation is the main source of myocardial regeneration [28]. The injury site regulates a variety of factors including retinoic acid [26], tgfb1 [29], pdgf [thirty], Shh [31], igf2b [31,32] and Notch signaling [three,24] to promote cardiomyocyte proliferation. In response, existing cardiomyocytes nigh the injury undergo express dedifferentiation with the disassembly of their sarcomeric structure and acquire immature phenotypes to facilitate mitosis before reconstituting the myocardium [28,33].
This study extends our investigation of the regeneration suppressive role of ARF in additional tissues, including the zebrafish centre and the mammalian digit. We demonstrate that ARF expression is upregulated during cardiac regeneration and slows the rate of morphological recovery, specifically impacting cardiomyocytes, neovascularization, and EMT, while not affecting epicardial proliferation. We also prove that ARF deficiency alone is insufficient to permit significant digit regeneration.
two. Materials and Methods
2.ane. Zebrafish and Mice
All experiments with zebrafish and mice were approved by the Institutional Animal Intendance and Use Commission of the University of California, San Francisco (ethical approving codes AN181101-01 and AN109021-03). As well, 3- to six-month-quondam wild type (WT) AB or transgenic AB zebrafish were used for all cardiac regeneration experiments. The transgenic hs:ARF and ARF:ARF fish were from in-crossed transgenic lines used previously [17]. 5-month-former C57BL/6J male WT and Arf knockout (KO) mice were used for digit regeneration experiments.
2.ii. Heat Shock Experiments
Heat shocks were delivered past housing fish in a water bathroom set to 37 °C with bidiurnal water exchanges. The h2o bath accomplished 37 °C within xv min, maintained that temperature for one h, and then passively cooled to fish room temperature (26–28 °C). An automated digital timer (Intermatic, Bound Grove, IL, USA) was used to turn on and off the water bath. For rut shock experiments, an initial estrus shock was delivered then hearts were injured three h later. Estrus shocks were subsequently delivered every 6 h for the duration of the experiment.
2.iii. Cryoinjury
Cardiac cryoinjury and eye autopsy were performed as previously described [7]. Sections with the well-nigh prominent cardiac injury from each sample were used. The injury site was approximated using Acid Fuchsin Orange 1000 (AFOG) and troponin staining looking for fibrin, collagen, and disorganized myocardium. Myocardial recovery was calculated equally the proportion of troponin infiltration into the demarcated injury site. Images were quantified using Adobe Photoshop CS6 software (Adobe, San Jose, CA, U.s.).
2.iv. Mouse Anesthesia
Anesthesia was induced using ii–three% isoflurane with 0.8–1.0 L O2 flow into an induction chamber, watching for slow abdominal breathing and testing depth past manus compression. Anesthesia was maintained using −two% isoflurane with 0.8–1.0 L O2 menses through a olfactory organ cone tubing. Isoflurane was decreased if there was agonal or oral fissure breathing. Animals were watched to full recovery after anesthesia and given analgesia if needed.
2.v. Digit Amputation
Mouse digit amputations were performed on a sterile field using a fresh 11-blade to make a make clean cut. Mice were placed in the lateral position with forceps used to isolate the digit of interest. Digits were amputated either proximal or distal to the nail bed to emulate unlike regenerative processes. Pressure was applied after amputation to stop bleeding as needed. Amputated digits were collected at 10, twenty, xxx, and 60 days post-amputation (dpa) for analysis. Digits were amputated at the metacarpophalangeal joint (MCPJ) for harvest.
2.6. Histology and Immunofluorescence
Zebrafish eye section: Dissected, done in phosphate buffered saline (PBS) containing 2 U/mL heparin and 0.i M KCl, fixed in iv% paraformaldehyde (PFA) 1 h, equilibrated in thirty% sucrose, frozen in optimal cutting temperature (October) blocks, sectioned at 10 µm, and stored at −80°C.
Mouse digit section: Dissected, fixed in 4% PFA overnight, washed in PBS 4–five times, decalcified in 0.4M ethylenediaminetetraacetic acid (EDTA) for two weeks (except for Micro-CT specimens) at 4 °C, washed in PBS 4–five times, frozen in 70% EtOH at −twenty °C for storage, dehydrated, cleared, embedded in alkane, sectioned at v µm.
AFOG staining: Rinsed in deionized (DI) water, fixed in Bouin'southward fluid (Thermo, Waltham, MA, United states) at 60 °C 2 h, rinsed in DI water xxx min, submerged in Weigert'south iron hematoxylin (Thermo) solution 10 min, rinsed in DI water five min, submerged in 3% phosphotungstic-phosphomolybdic acid solution (Thermo) 5 min, rinsed in DI water ii min, submerged in AFOG solution (5 g Methyl Blueish boiled in 1000 mL DI h2o, x g Orange Yard, 15 one thousand Acid Fuschin, pH adapted to i.09 by calculation HCl) 5 min, rinsed in DI h2o two min, dehydrated in titrated EtOH baths and xylene, and mounted with toluene.
Trichrome staining: Sections were deparaffinized in xylene, EtOH washes, and H2O wash prior to staining. Mallory's trichrome staining protocol was used.
Micro-CT: Mice were anesthetized and maintained with isoflurane for longitudinal X-ray and micro-CT studies of digit tip regeneration. 10-ray images were obtained every ii–three days using the scout view positioning function of a Scanco vivaCT-forty (Scanco, Brüttisellen, Switzerland) and total scans (55 kVp at 12.5 μm) were carried out on day 20.
Immunofluorescence: Done in PBS with 0.1% Tween (PBST) 5 min, blocked with serum-free protein cake (Dako) 1 h, primary antibodies in 5% goat serum antibody diluent at room temperature iv h, PBST v min, secondary antibodies in antibody diluent 1 h, PBST five min, mounted with Vectashield mounting medium with or without DAPI (Vector Laboratories, Burlingame, Ca, U.s.). Myocyte enhancer factor 2 (Mef2)/Proliferating prison cell nuclear antigen (PCNA) staining was performed on sections as described [26]. Mef2+ and Mef2+/PCNA+ cells were counted manually. Principal antibodies are bachelor in Table A1.
2.7. Quantitative Polymerase Chain Reaction (qPCR)
Cells were lysed with buffer RLT, and RNA was isolated using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. cDNA was produced from total RNA using the SuperScript III First Stand Synthesis Organization (Life Technologies, Carlsbad, CA, United states) per the manufacturer'due south protocol. Thermocycling and quantification were performed using the Mastercycler RealPlex 2 (Eppendorf, Hamburg, Germany). qPCR assays were performed on 10 ng of cDNA using 1.two µL of each primer (10 pmol/mL) and iTaq Universal SYBR Dark-green Supermix (Bio-Rad, Hercules, CA, USA) in a 12 µL total reaction volume. The PCR was performed for twoscore cycles with annealing temperatures of 55–60 °C and elongation times of 20 sec. The relative expression of individual genes compared to command groups was calculated by the delta delta bicycle threshold (ΔΔ-Ct) method with ribosomal protein S13 (RPS13) as the housekeeping gene. PCR primer sequences are available in Table A2.
2.8. Statistical Assay
Data are presented equally mean ± standard error. Statistical analyses were performed by using Stata Statistical Software, SE 12 (StataCorp, College Station, TX, U.s.a.). Statistical differences were analyzed by using the unpaired t-test, ANOVA, and Pearson's correlation coefficient. A p < 0.05 was set as the threshold for statistical significance.
3. Results
3.ane. Induced Expression of ARF Suppresses Cardiac Regeneration
To investigate whether human ARF assumes canonical functions in cardiac cells in vivo, we used fish in which ARF is expressed under the control of the heat shock poly peptide seventy inducible promoter (Tg(hsp70l:ARF) or hs:ARF) [17]. In the fin of hs:ARF fish, a rut shock regimen of 1 h at 37 °C drives pervasive expression of ARF in cells, with pinnacle ARF expression three h after heat daze [17]. qPCR confirmed that ARF mRNA is also expressed in the hearts of fish that underwent the aforementioned heat daze regimen (Figure 1a).
To evaluate the phenotypic impact of induced ARF expression on cardiac regeneration, the hearts of hs:ARF fish and non-transgenic WT clutchmates were cryoinjured 3 h afterwards estrus shock and were subsequently heat-shocked every 6 h to maintain ARF expression. hs:ARF and WT fish both tolerated heat shock without gross illness or bloodshed. Hearts were analyzed xv days post injury (dpi). ARF expression was associated with significantly less myocardial recovery within the injury site compared to WT controls that underwent heat stupor (Figure 1b). AFOG staining was used to visualize the injury site and the involved tissue types. With this technique, by 15 dpi in WT fish, the central office of the infarct area was replaced by a loose collagen network marked in blue, and a ruby fibrin layer formed in the inner margin of the wound was in the process of being replaced by myocytes at the edges, as previously described [six]. Compared to WT hearts that showed the expected regeneration phenotype, the hs:ARF hearts had a bulging disorganized collagen network, a less organized fibrin layer, and as well less muscle tissue infiltration of the injury site (Effigy 1b). The degree of myocardial recovery was better visualized by troponin immunostaining which showed significantly fewer troponin expressing myocytes in the hs:ARF injury site (Effigy 1b). When troponin presence was quantified equally a percentage of the injury site, WT recovery measured 57.8 ± five.1% compared with hs:ARF recovery, which measured 29.7 ± 1.iii%, a 48.7% reduction (p < 0.01) (Figure 1c).
iii.2. ARF Expressed under Control of the Endogenous Human ARF Promoter Suppresses Cardiac Regeneration
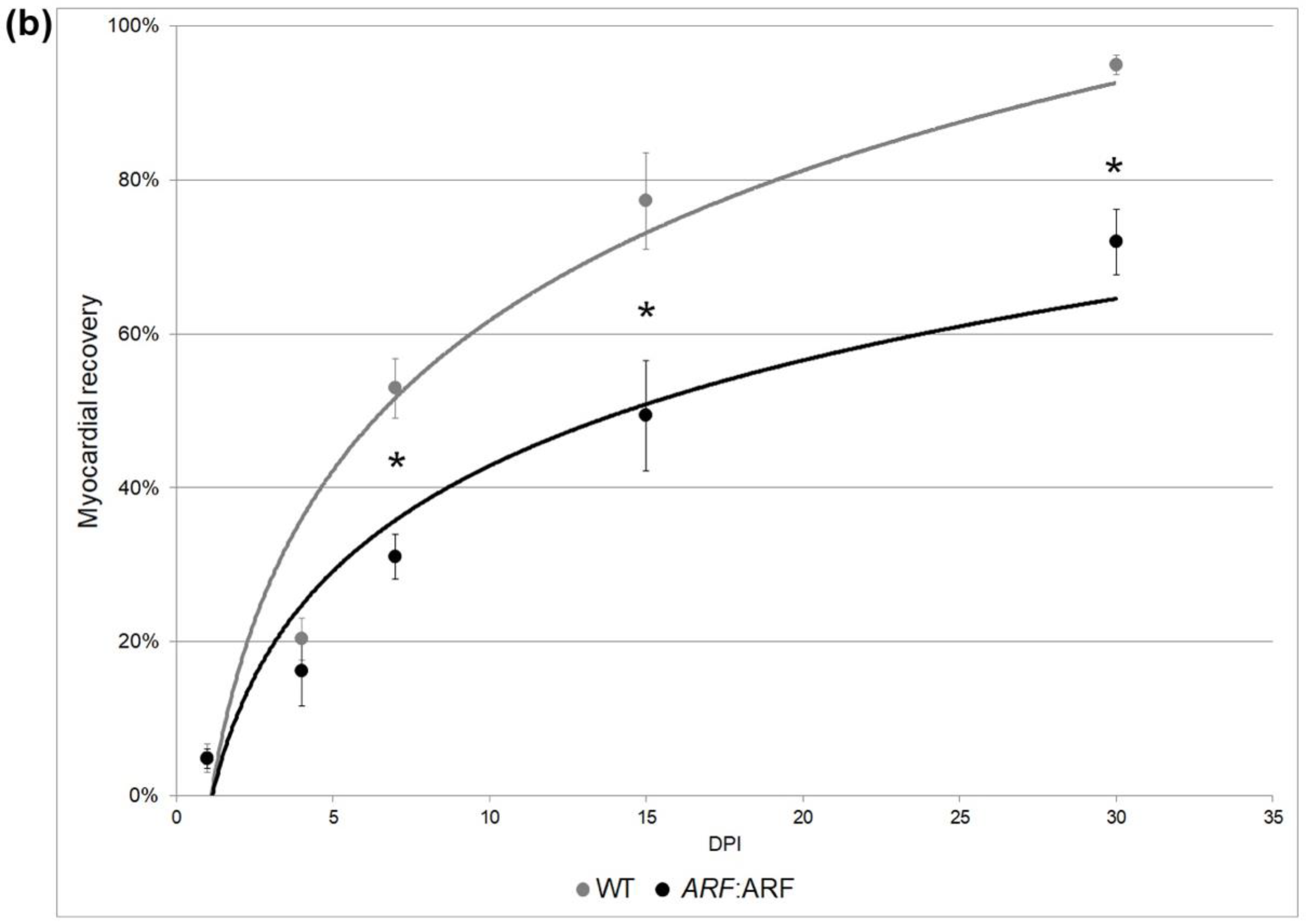
To empathize whether signals present in the regenerating heart would exist detected by ARF, we evaluated the impact of ARF expression when nether control of the native man ARF promoter using Tg(ARF:ARF), or ARF:ARF fish. The hearts of cryoinjured ARF:ARF and WT clutch mates were collected at 0, 1, four, 7, 11, 15, 30, and 45 dpi. The regeneration of ARF:ARF hearts was significantly delayed compared to WT fish (Figure ii). AFOG staining was used to qualify the progression of cardiac regeneration over time. WT hearts followed expected patterns of myocardial regeneration [6] with the formation of a loose collagen network and thin fibrin layer replacing necrotic myocardium by 7 dpi, and progressive myocardial replacement of damaged tissue inward from the healthy edges with complete scar tissue replacement by myocardium at 30 dpi (Figure 2a). By comparison, ARF:ARF hearts were characterized by a much more disorganized fibrin eolith on 7 dpi, and from that point forward, attained consistently less myocardial replacement of the injury site (Figure 2a). Even by 30 dpi when most WT hearts had fully recovered, in ARF:ARF fish, fibrin persisted in the injury site, which is typically cleared past 21 dpi (Figure 2a) [6].
When quantified by proportion of troponin inside the injury site, WT vs. ARF:ARF cardiomyocyte recovery measured 4.9 ± one.8% vs. 4.eight ± one.3%, 20.4 ± 2.viii% vs. sixteen.2 ± four.6%, 52.9 ± 3.9% vs. 31.0 ± 3.0%, 77.3 ± half-dozen.3% vs. 49.4 ± 7.two%, and 95.0 ± ane.3% vs. 72.0 ± 4.3% on 1, 4, 7, xv, and 30 dpi, respectively. ANOVA testing showed a significant difference betwixt WT and ARF:ARF myocardial recovery (p < 0.01). Stratifying by dpi, deficiencies in recovery of ARF:ARF hearts were ii.3% (p = 0.96), 20.4% (p = 0.47), 41.three% (p < 0.01), 36.1% (p = 0.05), and 24.3% (p < 0.01) at one, four, 7, 15, and 30 dpi, respectively (Figure 2b). In contrast to the fin, regeneration did methodically progress in ARF:ARF hearts despite the suppressed charge per unit. This differed from the amputated fins of some ARF:ARF fish which never fully regenerated regardless of time [17]. All ARF:ARF hearts were fully restored past 45 dpi compared to WT hearts which had regenerated by 30 dpi.
three.3. ARF Suppresses Cardiomyocyte Proliferation, Vascular Formation, and EMT
Cardiomyocyte proliferation during heart regeneration was quantified by immunostaining ARF:ARF and WT hearts at xi dpi with a cardiomyocyte nuclear marker (Mef2) and jail cell proliferation mark (PCNA) (Figure 3a). Cardiomyocyte proliferation index was calculated as a proportion of Mef2+/PCNA+ cells among all Mef2+ cells inside the injury site. The cardiomyocyte proliferation index was reduced past 46.half-dozen% (p = 0.01) in ARF:ARF hearts compared to WT (Figure 3b).
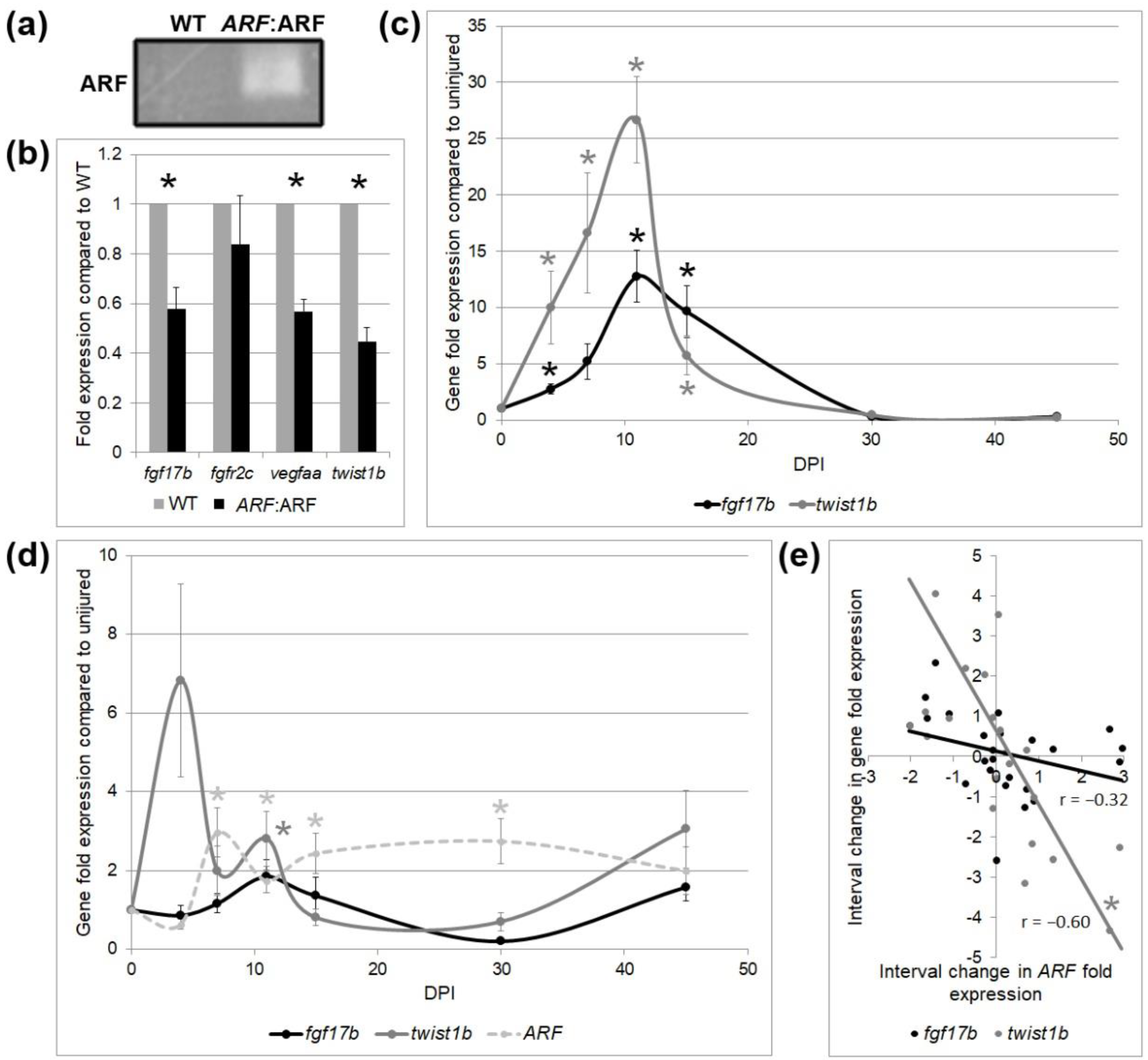
To further elucidate the cell types and processes involved in delayed cardiac regeneration in ARF:ARF fish, RNA of ARF:ARF and WT hearts were collected at 11 dpi to measure the expression of several tissue-specific regeneration associated genes. ARF mRNA expression in ARF:ARF cryoinjured hearts was confirmed by qPCR (Figure 4a). Depression levels of ARF mRNA were detected in uninjured hearts, which could indicate a low-level leakiness of the promoter, or elevated gratuitous E2F. A ligand-induced in myocardial cycling fibroblast growth factor 17b (fgf17b), a receptor-induced in epicardial cycling fibroblast growth factor receptor 2c (fgfr2c), and an endothelial growth factor expressed during vascular cycling vascular endothelial growth factor Aa (vegfaa) were measured [21,34]. fgf17b and vegfaa were reduced by 42% (p < 0.01) and 43% (p < 0.01), respectively, in ARF: ARF hearts compared to WT, reflective of decreases in myocardial regeneration, and vascular regeneration, respectively. There was no meaning difference in fgfr2c expression (p = 0.44) supporting that epicardial healing is unperturbed. twist1b, a mark for EMT [35], was reduced by 55% (p < 0.01) in ARF:ARF hearts, suggesting contradistinct EMT (Figure 4b).
ARF, fgf17b, and twist1b expression were also followed over time in regenerating ARF:ARF and WT hearts (Figure 4c). In WT hearts in the absenteeism of the ARF transgene, both fgf17b and twist1b rose steadily afterward cryoinjury earlier peaking on 11 dpi and tapering down to baseline levels by 30 dpi. The expression pattern mirrored the histological recovery (Effigy 2a), with the peak expression interval occurring between vii to¬ xv dpi, before returning to baseline. In contrast, ARF:ARF hearts had much more variable expression of fgf17b and twist1b (Figure 4d). fgf17b was never significantly increased compared to the injured baseline. twist1b was only significantly increased at 11 dpi (p = 0.04), and otherwise did non change significantly compared to uninjured baseline. ARF expression was tracked over the same menstruation in ARF:ARF fish. ARF expression was significantly elevated between 7–30 dpi (p < 0.04) compared to baseline, a consequent increase of ARF expression throughout the regeneration procedure before tapering off at 45 dpi when fifty-fifty ARF:ARF hearts had virtually fully recovered. Changes in ARF expression betwixt fourth dimension points roughly correlated with changed changes in fgf17b (r = −0.32, p = 0.xiii) and twist1b (r = −0.60, p < 0.01) expression (Figure 4e).
3.4. ARF Deficiency Is Bereft to Permit Digit Regeneration in Mice
Since our data collectively advise that ARF will impede naturally occurring epimorphic regeneration, nosotros asked the converse question of whether the absence of ARF alone would be permissive. To exam this, we examined murine digit regeneration in Arf KO mice. Every bit an initial pilot, Arf KO mice matched with WT controls underwent amputation at a distal or proximal location on the distal phalanx. Distal amputation was distal to the nail bed and tested whether Arf deficiency affected normal regrowth when the proximal nail bed stem cells remained nowadays. Proximal amputation was proximal to the nail bed to test whether Arf deficiency could permit epimorphic regeneration.
For amputations proximal to the smash bed, gross morphologic cess and histology showed that while healing occurred with scar, neither WT nor Arf deficient digits underwent epimorphic regeneration of the digit or nail (Effigy S1a). For distal digit amputations where the nail bed stem cells were intact, there was besides no significant difference between WT and Arf scarce digits in the extent or rate of regeneration (p = 0.56). Micro-computed tomography (micro-CT) likewise did non find substantial differences in bone regeneration. Arf deficient digits did comprise intramedullary sclerosis and disorganized periosteal osseous proliferation near the amputation margin compared to WT mice, just no mature ossification or cortication was noted past histology or micro-CT (Effigy S1b).
four. Discussion
In this extension of our initial findings that mammalian ARF is a suppressor of fin regeneration, we show this property of ARF to be similarly agile in the context of heart regeneration. Our findings imply that similarities exist among epimorphic regeneration processes that produce common cues able to be specifically sensed by ARF, inducing ARF expression and inhibition of regeneration. Despite differences in cell and tissue types and in mechanical processes of regeneration, there is a commonality of mail service-mitotic reentry into a proliferative land that is probable to be the common attribute of epimorphic regeneration, which in mammalian systems is restricted to the context of tumorigenesis. The findings imply similarities betwixt regeneration and tumorigenesis and point to possible incompatibilities of mammalian tumor suppressor mechanisms and epimorphic regeneration.
ARF expression specifically reduced cardiomyocyte proliferation by well-nigh 50% when expressed under the control of the hs promoter or the endogenous ARF promoter. Equally occurs after myocardial infarction, both WT and ARF:ARF hearts initially responded to cryoinjury with collagen formation and fibrin degradation over the injury site. In this astute phase of recovery during the first few days after injury, at that place was no pregnant difference between WT and ARF:ARF fish. The observation that differences became apparent betwixt 7–30 dpi further shows that ARF does not affect the astute initial wound healing response, similar to the case of wound healing in the fin [17]. Rather, ARF selectively suppresses regeneration responses including cardiomyocyte proliferation. This is farther demonstrated by the decreased cardiomyocyte proliferation index and fgf17b expression, suggesting that suppression of cardiomyocyte cycling is a primary, though not necessarily exclusive machinery by which ARF inhibits heart regeneration.
The selective suppression of cardiomyocytes is particularly highlighted past the observation that ARF expression appears to accept no touch on on epicardium as reflected by fgfr2c expression. A difference between cardiomyocyte and epicardial proliferation in cardiac regeneration subsequently injury relates to the fact that cardiomyocytes undergo limited dedifferentiation with the disassembly of their sarcomeric construction prior to proliferation [33], whereas epicardial cells speedily proliferate without dedifferentiation to grade a surrounding wound epidermis [21,22]. This suggests that ARF is specifically activated in cells undergoing dedifferentiation or at to the lowest degree post-mitotic reversal. The findings are consistent with our previous observations that ARF prevents dedifferentiation in muscle cells in civilization [16] and is expressed in the proliferating blastema of an injured fin but not the surrounding epithelial tissue [17].
The triggers for ARF activation are farther elucidated by the impact of ARF on EMT. EMT is a process during which polarized epithelial cells undergo biochemical changes to presume a mesenchymal phenotype [36]. In cardiac regeneration, EMT is of import for generating new coronary vasculature via the epicardium [22]. Despite epicardial proliferation being unaffected in ARF:ARF fish, EMT is significantly reduced as reflected by twist1b expression. Since a meaning portion of the new coronary vasculature is generated by EMT, information technology follows that vegfaa would as well be expected to exist reduced every bit observed in our study. This suggests that ARF is activated to suppress both dedifferentiating cells and cells undergoing EMT. By dissimilarity, fgfr2c expressing epicardial cells, while providing critical signals for cardiac regeneration, exercise non undergo significant structural or cell-type modify [23], and therefore we hypothesize they are thus non impacted by ARF during proliferation. Important considerations in future enquiry should consider confirming the inferences of factor expression morphologically in situ via measures such as directly visualizing neovascularization or recording cardiac part tests to better narrate the functional touch of ARF.
Since dedifferentiation and metaplasia are common characteristics of tumorigenesis, our new findings further reinforce prior evidence that ARF, while a critical tumor suppressor, simultaneously opposes regeneration functions [nine,17,37]. Despite being able to distinguish developmental from regenerative contexts [17], ARF is unable to distinguish epimorphic regeneration and tumorigenesis. However, an test of regeneration in developed mouse digits, in which blastema formation does non occur, showed no significant change in the absence of Arf. This is not surprising since regeneration is a circuitous process unlikely to be enabled by a single gene. Furthermore, since our experiments in the fish fin and heart propose that ARF interferes with proliferation in established blastemas, ARF would be expected to assume relevance in the mammalian context when regeneration progresses to the blastema stage.
Supplementary Materials
Author Contributions
Conceptualization, S.Fifty., R.H., C.Yard. and J.H.P.; methodology, Due south.50., R.H., S.T., C.G. and J.H.P.; validation, South.50.; formal analysis, Due south.L.; investigation, Due south.L. and C.G.; resource, S.T. and J.H.P.; data curation, S.L.; writing—original draft preparation, S.L.; writing—review and editing, J.H.P.; visualization, South.L.; supervision, Due south.T. and J.H.P.; project administration, S.L and J.H.P.; funding acquisition, S.L. and J.H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by funds from the Sandler Foundation Opportunity Award, Program for Quantum Biomedical Research to J.H.P., the UCSF Department of Surgery, and the UCSF Yearlong Enquiry Program to S.L.
Acknowledgments
The authors would like to acknowledge Zoya Qureshy assist with data collection, and Rina Patel, Medico for micro-CT interpretation.
Conflicts of Involvement
The authors declare no conflict of involvement. The funders had no role in the blueprint of the study; in the drove, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
Table A1. Principal antibodies.
Table A1. Principal antibodies.
| Antigen | Host Species | Company | Cat. No. | Dilution |
|---|---|---|---|---|
| Troponin | Mouse | Invitrogen | MA5-12960 | i:1000 |
| Mef2 | Rabbit | Abcam | ab64644 | one:50 |
| PCNA | Mouse | Jail cell Signaling | 2586T | i:100 |
Table A2. qPCR Primers.
| Gene | NCBI ID | Forward | Contrary |
|---|---|---|---|
| B-Actin | NM_131031.1 | TTCACCACCACAGCCGAAAGA | TACCGCAAGATTCCATACCCA |
| ARF | NM_058195.3 | ATGGTGCGCAGGTTCTTGGTGA | CACCACCAGCGTGTCCAGGAAG |
| Fgf17b | NM_214808.one | GCGGACGACGGAAACTCTTACG | CTCGAAGTGTTTTGGCTGCTCT |
| Fgfr2c | NM_001243004.ane | CGGCAGGTGTGAACACTACGG | CTCCGGCGAGTGGTGATTCTG |
| Vegfaa | NM_001110349.2 | GTGCAGGATGCTGTAATGATGAGG | AATTATGCTGCGATACGCGTTG |
| Twist1b | NM_001017820.1 | AGGTTCTACAGAGTGACGAGC | GCACAGGATTCGAACTAGAGG |
References
- Poss, Grand.D. Advances in understanding tissue regenerative chapters and mechanisms in animals. Nat. Rev. Genet. 2010, 11, 710–722. [Google Scholar] [CrossRef]
- Poss, G.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Scientific discipline 2002, 298, 2188–2190. [Google Scholar] [CrossRef]
- Raya, A.; Koth, C.Thou.; Büscher, D.; Kawakami, Y.; Itoh, T.; Raya, R.M.; Sternik, G.; Tsai, H.-J.; Rodríguez-Esteban, C.; Izpisua Belmonte, J.C. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc. Natl. Acad. Sci. The states 2003, 100, 11889–11895. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Panáková, D.; Kikuchi, Yard.; Holdway, J.E.; Gemberling, M.; Burris, J.S.; Singh, Southward.P.; Dickson, A.Fifty.; Lin, Y.F.; Sabeh, 1000.K.; et al. The regenerative capacity of zebrafish reverses cardiac failure acquired by genetic cardiomyocyte depletion. Development 2011, 138, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Parente, V.; Balasso, S.; Pompilio, Thou.; Verduci, L.; Colombo, G.I.; Milano, G.; Guerrini, U.; Squadroni, L.; Cotelli, F.; Pozzoli, O.; et al. Hypoxia/reoxygenation cardiac injury and regeneration in zebrafish adult centre. PLoS 1. 2013, viii, e53748. [Google Scholar] [CrossRef] [PubMed]
- Chablais, F.; Veit, J.; Rainer, G.; Jaźwińska, A. The zebrafish centre regenerates later cryoinjury-induced myocardial infarction. BMC Dev. Biol. 2011, 11, 21. [Google Scholar] [CrossRef]
- González-Rosa, J.Yard.; Martín, V.; Peralta, M.; Torres, M.; Mercader, N. Extensive scar germination and regression during heart regeneration after cryoinjury in zebrafish. Development 2011, 138, 1663–1674. [Google Scholar] [CrossRef]
- Schnabel, K.; Wu, C.-C.; Kurth, T.; Weidinger, Thousand. Regeneration of cryoinjury induced necrotic center lesions in zebrafish is associated with epicar-dial activation and cardiomyocyte proliferation. PLoS ONE 2011, 6, e18503. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, J.H.; Blau, H.M. Tumor suppressors: Enhancers or suppressors of regeneration? Evolution 2013, 140, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Flicek, P.; Amode, G.R.; Barrell, D.; Beal, Thousand.; Billis, K.; Brent, S.; Carvalho-Silva, D.; Clapham, P.; Coates, G.; Fitzgerald, S.; et al. Ensembl 2014. Nucleic Acids Res. 2014, 42, D749–D755. [Google Scholar] [CrossRef] [PubMed]
- Karolchik, D.; Barber, Thou.P.; Casper, J.; Clawson, H.; Cline, Grand.S.; Diekhans, M.; Dreszer, T.R.; Fujita, P.A.; Guruvadoo, L.; Haeussler, M.; et al. The UCSC Genome Browser database: 2014 update. Nucleic Acids Res. 2014, 42, D764–D770. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.; Pomerantz, J.H.; DePinho, R.A. The INK4a/ARF tumor suppressor: One factor—ii products—ii pathways. Trends Biochem. Sci. 1998, 23, 291–296. [Google Scholar] [CrossRef]
- Lowe, S.W.; Sherr, C.J. Tumor suppression by Ink4a–arf: Progress and puzzles. Current Opinion in Genetics & Development. Curr. Opin. Genet. Dev. 2003, 13, 77–83. [Google Scholar] [PubMed]
- Pomerantz, J.H.; Schreiber-Agus, N.; Liégeois, N.J.; Silverman, A.; Alland, 50.; Chin, L.; Potes, J.; Chen, One thousand.; Orlow, I.; Lee, H.Due west.; et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Prison cell 1998, 92, 713–723. [Google Scholar] [CrossRef]
- Weber, J.D.; Taylor, L.J.; Roussel, Thou.F.; Sherr, C.J.; Bar-Sagi, D. Nucleolar arf sequesters Mdm2 and activates p53. Nat. Cell Biol. 1999, 1, 20–26. [Google Scholar] [CrossRef]
- Pajcini, K.V.; Corbel, Southward.Y.; Pomerantz, J.H.; Blau, H.One thousand.; Sage, J.; Pomerantz, J.H.; Blau, H.M. Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian musculus. Prison cell Stem Cell 2010, 7, 198–213. [Google Scholar] [CrossRef]
- Hesse, R.Chiliad.; Kouklis, Chiliad.M.; Ahituv, N.; Pomerantz, J.H. The human ARF tumor suppressor senses blastema activeness and suppresses epimorphic tissue regeneration. Elife 2015, iv, e07702. [Google Scholar] [CrossRef]
- Knopf, F.; Hammond, C.; Chekuru, A.; Kurth, T.; Hans, Southward.; Weber, C.Westward.; Mahatma, G.; Fisher, S.; Make, M.; Schulte-Merker, S.; et al. Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev. Prison cell 2011, xx, 713–724. [Google Scholar] [CrossRef]
- Tu, Southward.; Johnson, Southward.50. Fate brake in the growing and regenerating zebrafish fin. Dev. Cell 2011, twenty, 725–732. [Google Scholar] [CrossRef]
- Bertero, A.; Murry, C.Due east. Hallmarks of cardiac regeneration. Nat. Rev. Cardiol. 2018, 15, 579–580. [Google Scholar] [CrossRef]
- Kikuchi, K.; Holdway, J.E.; Werdich, A.A.; Anderson, R.M.; Fang, Y.; Egnaczyk, 1000.F.; Evans, T.; Macrae, C.A.; Stainier, D.Y.; Poss, Thousand.D. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 2010, 464, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Lepilina, A.; Coon, A.N.; Kikuchi, K.; Holdway, J.East.; Roberts, R.W.; Burns, C.Thou.; Poss, Chiliad.D. A dynamic epicardial injury response supports progenitor cell activity during zebrafish middle regeneration. Jail cell 2006, 127, 607–619. [Google Scholar] [CrossRef]
- Cao, J.; Poss, K.D. The epicardium equally a hub for heart regeneration. Nat. Rev. Cardiol. 2018, 15, 631–647. [Google Scholar] [CrossRef]
- Zhao, L.; Borikova, A.Fifty.; Ben-Yair, R.; Guner-Ataman, B.; MacRae, C.A.; Lee, R.T.; Burns, C.One thousand.; Burns, C.Due east. Notch signaling regulates cardiomyocyte proliferation during zebrafish centre regeneration. Proc. Natl. Acad. Sci. U.s. 2014, 111, 1403–1408. [Google Scholar] [CrossRef]
- Zhou, B.; Accolade, L.B.; He, H.; Ma, Q.; Oh, J.H.; Butterfield, C.; Lin, R.Z.; Melero-Martin, J.Grand.; Dolmatova, E.; Duffy, H.S.; et al. Developed mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Investig. 2011, 121, 1894–1904. [Google Scholar] [CrossRef]
- Kikuchi, K.; Holdway, J.East.; Major, R.J.; Blum, N.; Dahn, R.D.; Begemann, Thousand.; Poss, G.D. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 2011, xx, 397–404. [Google Scholar] [CrossRef]
- Zhang, R.; Han, P.; Yang, H.; Ouyang, K.; Lee, D.; Lin, Y.F.; Ocorr, One thousand.; Kang, Chiliad.; Chen, J.; Stainier, D.Y.; et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature 2013, 498, 497–501. [Google Scholar] [CrossRef]
- Kikuchi, K. Dedifferentiation, Transdifferentiation, and Proliferation: Mechanisms Underlying Cardiac Musculus Regeneration in Zebrafish. Curr. Pathobiol. Rep. 2015, three, 81–88. [Google Scholar] [CrossRef]
- Chablais, F.; Jazwinska, A. The regenerative capacity of the zebrafish heart is dependent on TGFβ signaling. Development 2012, 139, 1921–1930. [Google Scholar] [CrossRef]
- Lien, C.L.; Schebesta, M.; Makino, South.; Weber, G.J.; Keating, Thou.T. Gene expression analysis of zebrafish centre regeneration. PLoS Biol. 2006, four, e260. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.Y.; Gemberling, M.; Wang, J.; Holdway, J.East.; Shen, M.C.; Karlstrom, R.O.; Poss, K.D. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Evolution 2013, 140, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Harrison, Thou.R.; Osorio, A.; Kim, J.; Baugh, A.; Duan, C.; Sucov, H.K.; Lien, C.L. Igf Signaling is Required for Cardiomyocyte Proliferation during Zebrafish Middle Development and Regeneration. PLoS Ane 2013, 8, e67266. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.; Sleep, E.; Raya, M.; Marti, Thou.; Raya, A.; Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef]
- Marín-Juez, R.; Marass, M.; Gauvrit, Due south.; Rossi, A.; Lai, South.; Materna, S.C.; Blackness, B.L.; Stainier, D. Fast revascularization of the injured expanse is essential to back up zebrafish heart regeneration. Proc. Natl. Acad. Sci. U.s. 2016, twoscore, 11237–11242. [Google Scholar] [CrossRef]
- Kim, J.; Wu, Q.; Zhang, Y.; Wiens, K.G.; Huang, Y.; Rubin, N.; Shimada, H.; Handin, R.I.; Chao, 1000.Y.; Tuan, T.L.; et al. PDGF signaling is required for epicardial function and blood vessel germination in regenerating zebrafish hearts. Proc. Natl. Acad. Sci. USA 2010, 40, 17206–17210. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Sharpless, Due north.East.; Depinho, R.A. How stalk cells age and why this makes us grow old. Nat. Rev. Mol. Prison cell Biol. 2007, 8, 703–713. [Google Scholar] [CrossRef]
Figure 1. Induced Alternative Reading Frame (ARF) expression in hs:ARF fish suppresses cardiac regeneration. (a) Representative polymerase concatenation reaction (PCR) product of ARF expression from iii experimental replicates of wild type (WT) and hs:ARF eye RNA on 15 dpi. The product run on gel electrophoresis shows ARF expression nowadays in hs:ARF fish while absent in WT fish. (b) Acid Fuchsin Orangish G (AFOG) and troponin staining of WT and hs:ARF middle cryosections on 15 days post-injury (dpi). The dotted lines demarcate the injury site. Red stains are for fibrin. Blue stains are for collagen. Brown stains are for muscle. Green stains are for troponin. (c) Myocardial recovery measured by troponin infiltration into the injury site. Infiltration quantified by imaging software shows decreased troponin in the injury site of hs:ARF fish compared to WT fish. N = 8 hearts. Results are shown as hateful ± standard error. The * represents a statistically significant divergence.
Effigy 1. Induced Alternative Reading Frame (ARF) expression in hs:ARF fish suppresses cardiac regeneration. (a) Representative polymerase chain reaction (PCR) production of ARF expression from iii experimental replicates of wild blazon (WT) and hs:ARF heart RNA on fifteen dpi. The production run on gel electrophoresis shows ARF expression nowadays in hs:ARF fish while absent in WT fish. (b) Acrid Fuchsin Orange G (AFOG) and troponin staining of WT and hs:ARF heart cryosections on 15 days mail-injury (dpi). The dotted lines demarcate the injury site. Red stains are for fibrin. Blue stains are for collagen. Brownish stains are for muscle. Green stains are for troponin. (c) Myocardial recovery measured by troponin infiltration into the injury site. Infiltration quantified by imaging software shows decreased troponin in the injury site of hs:ARF fish compared to WT fish. N = viii hearts. Results are shown as mean ± standard error. The * represents a statistically significant difference.
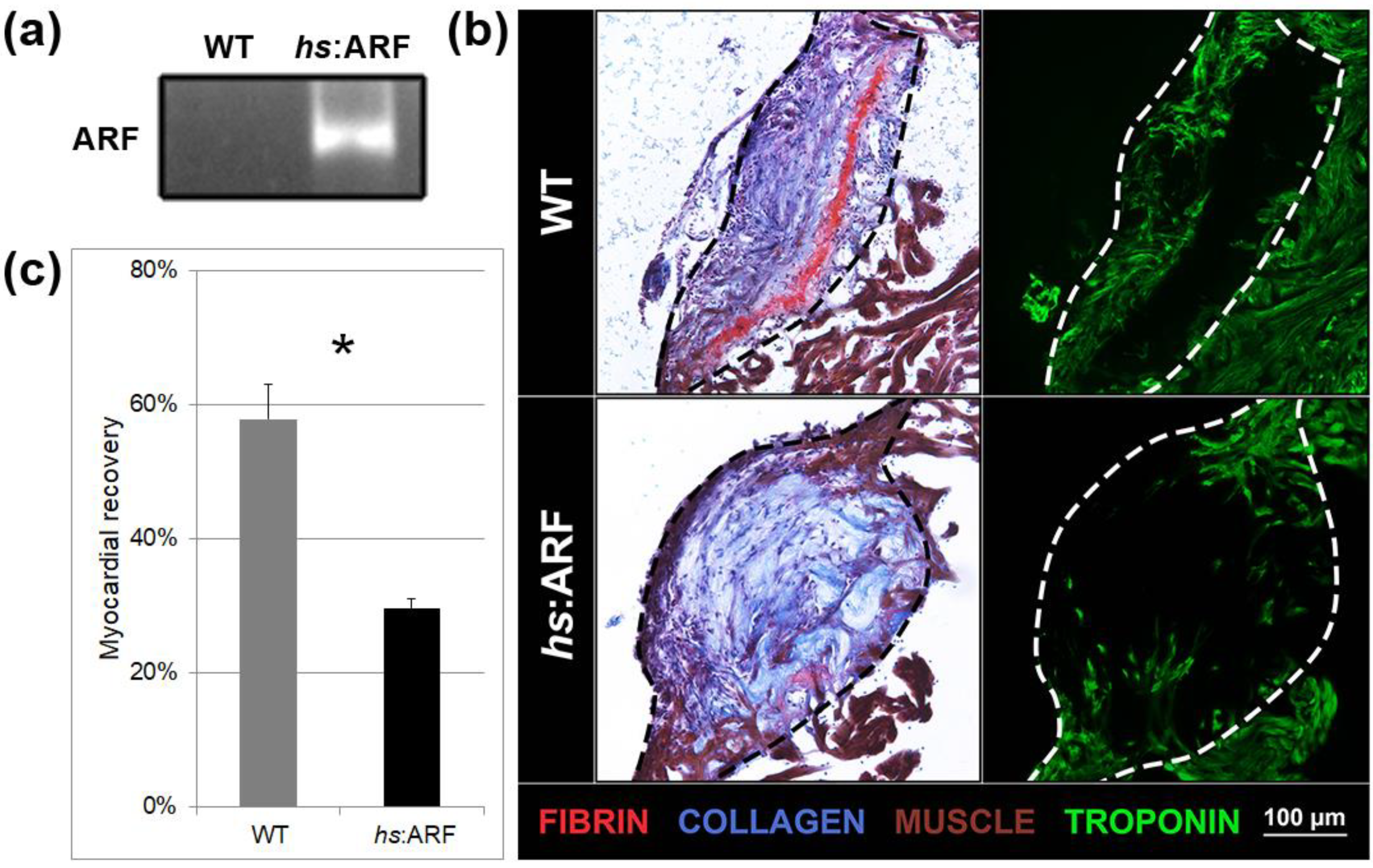
Figure 2. ARF expressed under control of the endogenous man ARF promoter in ARF:ARF fish suppresses cardiac regeneration over time. (a) AFOG and troponin staining of WT and ARF:ARF center cryosections at i, 7, 15, and 30 dpi. The dotted lines demarcate the injury site. Red stains are for fibrin. Blue stains are for collagen. Brown stains are for muscle. Greenish stains are for troponin. (b) Myocardial recovery measured by troponin infiltration into the injury site. Infiltration quantified by imaging software shows decreased troponin in the injury site of ARF:ARF fish compared to WT fish from 7 dpi onward. N = 49 hearts. The grey and blackness lines are logarithmic approximations of the WT and ARF:ARF eye recovery trends respectively. Results are shown as mean ± standard fault. The * represents a statistically significant divergence.
Figure ii. ARF expressed under control of the endogenous human ARF promoter in ARF:ARF fish suppresses cardiac regeneration over time. (a) AFOG and troponin staining of WT and ARF:ARF heart cryosections at 1, seven, 15, and thirty dpi. The dotted lines demarcate the injury site. Reddish stains are for fibrin. Blue stains are for collagen. Brown stains are for muscle. Light-green stains are for troponin. (b) Myocardial recovery measured past troponin infiltration into the injury site. Infiltration quantified by imaging software shows decreased troponin in the injury site of ARF:ARF fish compared to WT fish from 7 dpi onward. N = 49 hearts. The grey and black lines are logarithmic approximations of the WT and ARF:ARF heart recovery trends respectively. Results are shown as mean ± standard error. The * represents a statistically significant difference.

Effigy three. ARF expressed in ARF:ARF fish suppresses cardiomyocyte proliferation. (a) Myocyte enhancer factor 2 (Mef2), proliferating cell nuclear antigen (PCNA), and four′,6-diamidino-2-phenylindole (DAPI) staining in WT and ARF:ARF fish at 11 dpi. Bluish stains are for all nuclei. Ruddy stains are for Mef2, a cardiomyocyte nuclear marker. Greenish stains are for PCNA, a cell cycling mark. (b) The cardiomyocyte (CM) proliferation index shows decreased cardiomyocyte cycling in ARF:ARF fish. The CM proliferation index was calculated by counting Mef2+/PCNA+ cells out of total Mef2+ cells in the injury zone. N = 8 hearts. Results are shown as hateful ± standard fault. The * represents a statistically significant difference.
Figure three. ARF expressed in ARF:ARF fish suppresses cardiomyocyte proliferation. (a) Myocyte enhancer factor two (Mef2), proliferating prison cell nuclear antigen (PCNA), and four′,six-diamidino-2-phenylindole (DAPI) staining in WT and ARF:ARF fish at eleven dpi. Bluish stains are for all nuclei. Red stains are for Mef2, a cardiomyocyte nuclear marker. Dark-green stains are for PCNA, a cell cycling marking. (b) The cardiomyocyte (CM) proliferation alphabetize shows decreased cardiomyocyte cycling in ARF:ARF fish. The CM proliferation index was calculated by counting Mef2+/PCNA+ cells out of total Mef2+ cells in the injury zone. Due north = viii hearts. Results are shown as mean ± standard mistake. The * represents a statistically significant difference.
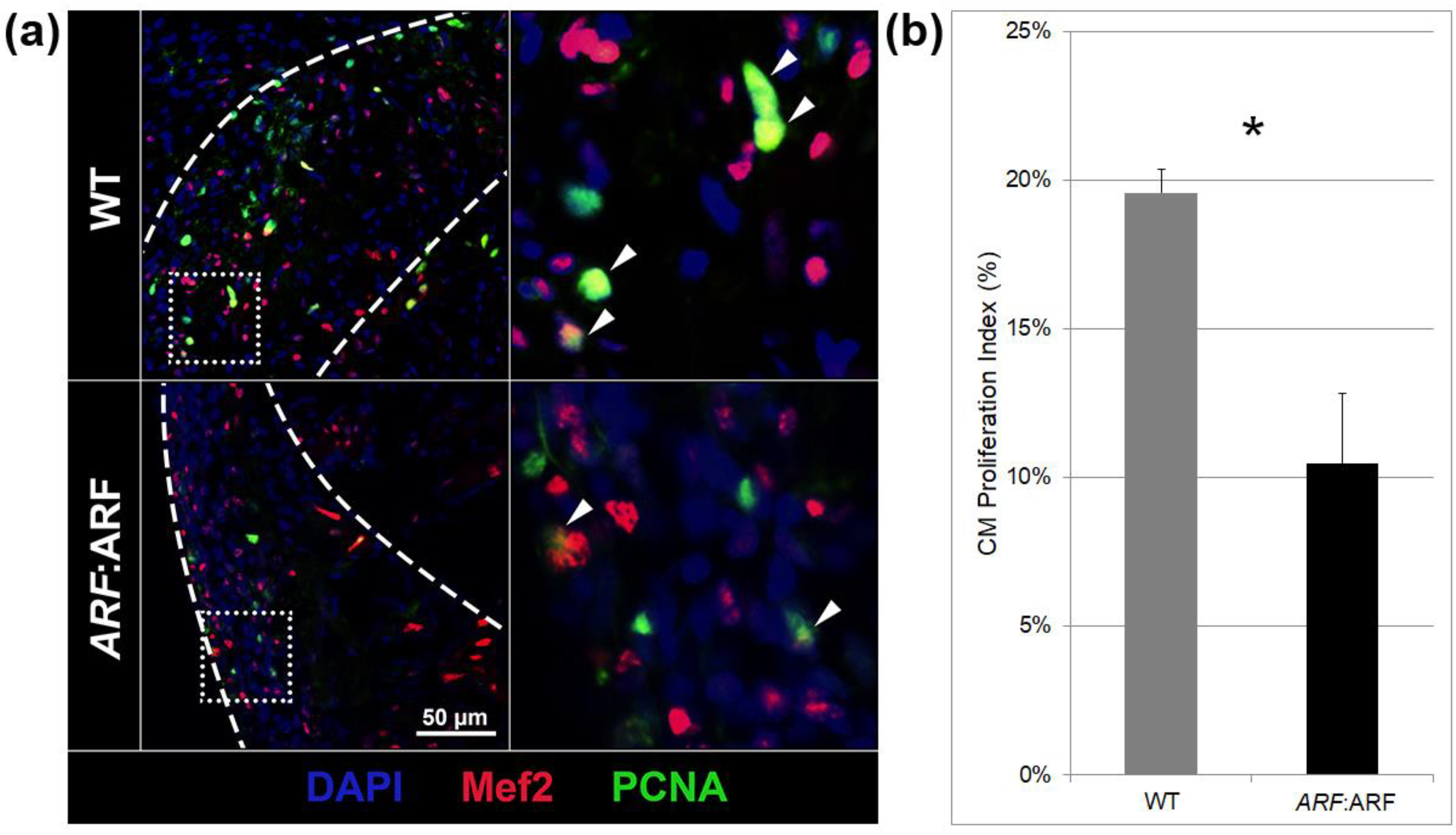
Figure 4. Tissue-specific gene expression by qPCR in WT and ARF:ARF fish over time. (a) Representative PCR product of ARF expression from three experimental replicates of WT and ARF:ARF heart RNA. The product run on gel electrophoresis shows ARF expression present in ARF:ARF fish while absent-minded in WT fish at 11 dpi. (b) qPCR of fibroblast growth factor 17b (fgf17b), fibroblast growth factor receptor 2c (fgfr2c), vascular endothelial growth factor Aa (vegfaa), and twist1b mRNA expression for WT and ARF:ARF fish at 11 dpi. Results show a significant subtract in the expression of fgf17b, vegfaa, and twist1b in ARF:ARF fish. No significant difference occurred for fgfr2c. Due north = 8 hearts. (c) fgf17b and twist1b mRNA expression for WT fish over time compared to uninjured WT command. fgf17b and twist1b rise steadily afterward cryoinjury and peak past 11 dpi earlier tapering down to the uninjured baseline by thirty dpi. Due north = 18 hearts. (d) fgf17b, twist1b, and ARF mRNA expression for ARF:ARF fish over time compared to uninjured ARF:ARF control. ARF is significantly elevated higher up the uninjured baseline from vii–30 dpi. fgf17b is never elevated higher up the uninjured baseline. twist1b expression is simply elevated above the uninjured baseline on 11 dpi. N = 24 hearts. (e) Correlation between interval alter in ARF expression with changes in fgf17b and twist1b expression at whatever fourth dimension bespeak. ARF expression trended toward inverse correlation with fgf17b expression and was inversely correlated with twist1b expression. Northward = 24 hearts. Results are shown as mean ± standard fault. The * represents a statistically significant difference.
Figure 4. Tissue-specific cistron expression past qPCR in WT and ARF:ARF fish over time. (a) Representative PCR product of ARF expression from iii experimental replicates of WT and ARF:ARF eye RNA. The product run on gel electrophoresis shows ARF expression present in ARF:ARF fish while absent in WT fish at 11 dpi. (b) qPCR of fibroblast growth factor 17b (fgf17b), fibroblast growth factor receptor 2c (fgfr2c), vascular endothelial growth factor Aa (vegfaa), and twist1b mRNA expression for WT and ARF:ARF fish at eleven dpi. Results show a significant decrease in the expression of fgf17b, vegfaa, and twist1b in ARF:ARF fish. No significant difference occurred for fgfr2c. North = viii hearts. (c) fgf17b and twist1b mRNA expression for WT fish over time compared to uninjured WT control. fgf17b and twist1b ascent steadily after cryoinjury and pinnacle by 11 dpi before tapering downwards to the uninjured baseline by 30 dpi. N = xviii hearts. (d) fgf17b, twist1b, and ARF mRNA expression for ARF:ARF fish over time compared to uninjured ARF:ARF control. ARF is significantly elevated to a higher place the uninjured baseline from 7–30 dpi. fgf17b is never elevated in a higher place the uninjured baseline. twist1b expression is merely elevated in a higher place the uninjured baseline on 11 dpi. N = 24 hearts. (e) Correlation between interval modify in ARF expression with changes in fgf17b and twist1b expression at whatsoever fourth dimension point. ARF expression trended toward inverse correlation with fgf17b expression and was inversely correlated with twist1b expression. Due north = 24 hearts. Results are shown as hateful ± standard error. The * represents a statistically significant difference.
© 2020 past the authors. Licensee MDPI, Basel, Switzerland. This article is an open up admission article distributed under the terms and conditions of the Artistic Commons Attribution (CC Past) license (http://creativecommons.org/licenses/by/iv.0/).
Source: https://www.mdpi.com/2073-4425/11/6/666/htm
 ,
,
0 Response to "​ according to brown, et al., what is the culture of honor hypothesis?"
Post a Comment